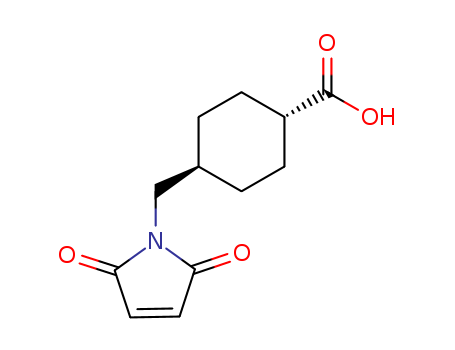
69907-67-1
- Product Name:Trans-4-(Maleimidomethyl)cyclohexanecarboxylic Acid
- Molecular Formula:C12H15NO4
- Purity:99%
- Molecular Weight:237.255
Product Details
pd_meltingpoint:144 °C(Solv: benzene (71-43-2))
Purity:99%
Quality Factory Supply High Purity 99% 69907-67-1, Cheapest Price Trans-4-(Maleimidomethyl)cyclohexanecarboxylic Acid
- Molecular Formula:C12H15NO4
- Molecular Weight:237.255
- Melting Point:144 °C(Solv: benzene (71-43-2))
- Boiling Point:433.6±18.0 °C(Predicted)
- PKA:4.80±0.10(Predicted)
- Density:1.329
Trans-4-(Maleimidomethyl)cyclohexanecarboxylic Acid(Cas 69907-67-1) Usage
|
Description |
Trans-4-(Maleimidomethyl) cyclohexanecarboxylic Acid is a kind of carboxylate, being well known for its N-hydroxysuccinimide ester derivative (SMCC), which is a useful cross-linking reagent. SMCC is widely used for the generation of stable maleimide-activated carrier proteins, enzyme immunoconjugates and hapten carrier molecule conjugates. It can be used for the coupling of a peptide to an amino group. |
InChI:InChI=1S/C12H15NO4/c14-10-5-6-11(15)13(10)7-8-1-3-9(4-2-8)12(16)17/h5-6,8-9H,1-4,7H2,(H,16,17)/t8-,9-
69907-67-1 Relevant articles
CYCLIC COMPOUNDS AND METHODS OF MAKING AND USING
-
Page/Page column 110, (2020/10/19)
Disclosed are compounds and methods for ...
Chemoselective Peptide Cyclization and Bicyclization Directly on Unprotected Peptides
Zhang, Yue,Zhang, Qing,Wong, Clarence T. T.,Li, Xuechen
supporting information, p. 12274 - 12279 (2019/08/20)
Cyclic peptides are drawing wide attenti...
PHARMACEUTICAL COMPOSITIONS COMPRISING MACROLIDE DIASTEREOMERS, METHODS OF THEIR SYNTHESIS AND THERAPEUTIC USES
-
Paragraph 00103, (2015/03/16)
The disclosure relates to compositions c...
Azide-alkyne cycloaddition for universal post-synthetic modifications of nucleic acids and effective synthesis of bioactive nucleic acid conjugates
Su, Yu-Chih,Lo, Yu-Lun,Hwang, Chi-Ching,Wang, Li-Fang,Wu, Min Hui,Wang, Eng-Chi,Wang, Yun-Ming,Wang, Tzu-Pin
, p. 6624 - 6633 (2014/08/18)
The regioselective post-synthetic modifi...
69907-67-1 Process route
-
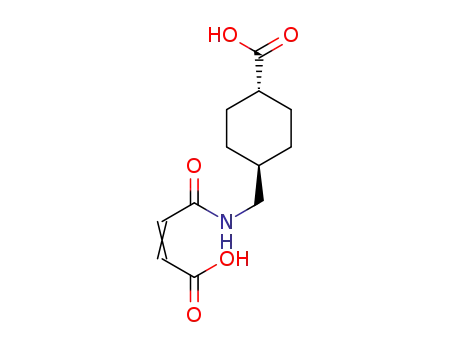
- 953817-12-4
C12H17NO5

-
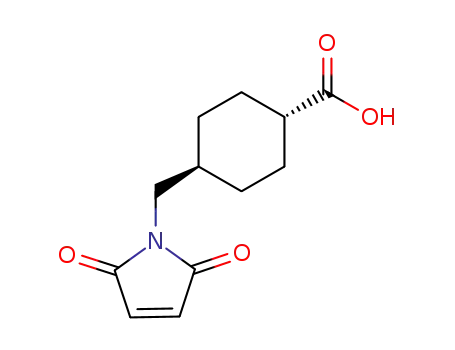
- 69907-67-1,64987-82-2
(1r,4r)-4-((2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)methyl)cyclohexane-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
With sodium acetate; acetic anhydride; at 120 ℃; for 3h; Sealed tube;
|
7 g |
-
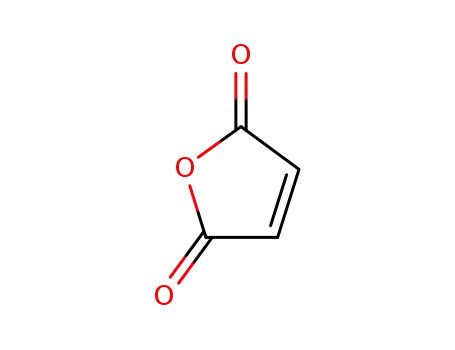
- 108-31-6
maleic anhydride

-
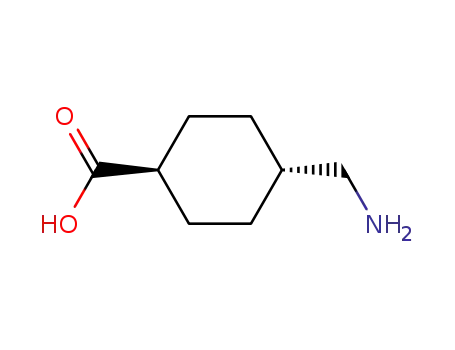
- 1197-18-8
trans-4-aminomethyl-cyclohexyl-carboxylic acid

-

- 69907-67-1,64987-82-2
(1r,4r)-4-((2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)methyl)cyclohexane-1-carboxylic acid
| Conditions | Yield |
|---|---|
|
In N,N-dimethyl-formamide; for 6h;
|
|
|
maleic anhydride; trans-4-aminomethyl-cyclohexyl-carboxylic acid; In N,N-dimethyl-formamide; at 20 ℃; for 3h;
With 2,4,6-trimethyl-pyridine; In N,N-dimethyl-formamide; at 0 ℃;
|
|
|
maleic anhydride; trans-4-aminomethyl-cyclohexyl-carboxylic acid; With acetic acid;
In toluene; Dean-Stark; Reflux;
|
|
|
maleic anhydride; trans-4-aminomethyl-cyclohexyl-carboxylic acid; With acetic acid;
In toluene; Dean-Stark; Reflux;
|
69907-67-1 Upstream products
-
108-31-6

maleic anhydride
-
1197-18-8

trans-4-aminomethyl-cyclohexyl-carboxylic acid
-
69907-68-2
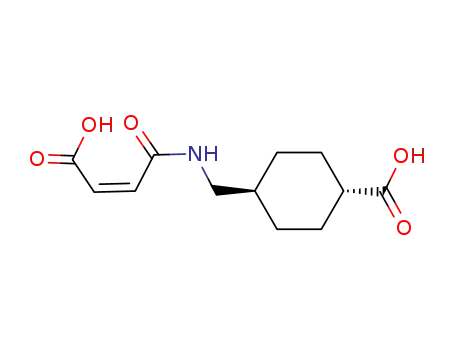
trans-4-([(2Z)-3-carboxyprop-2-enoyl]aminolmethyl)cyclohexanecarboxylic acid
-
953817-12-4

C12H17NO5
69907-67-1 Downstream products
-
71875-81-5
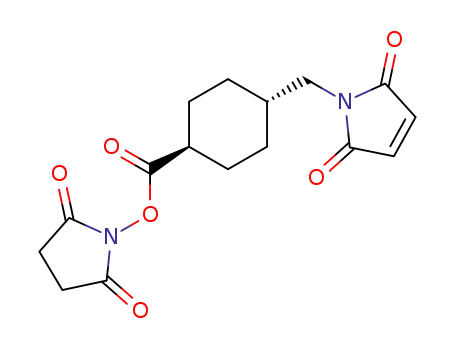
4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester
Relevant Products
-
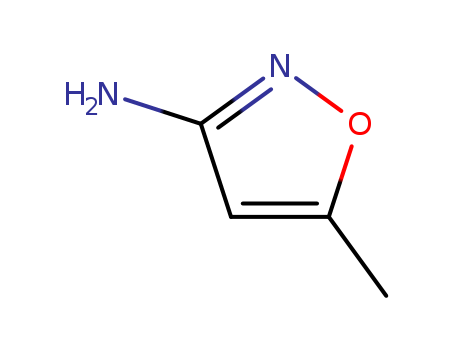
3-Amino-5-methylisoxazole
CAS:1072-67-9
-
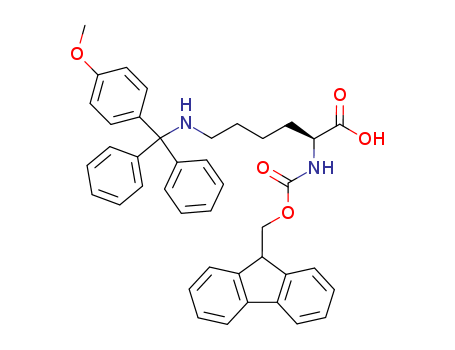
Fmoc-Lys(Mmt)-OH
CAS:159857-60-0