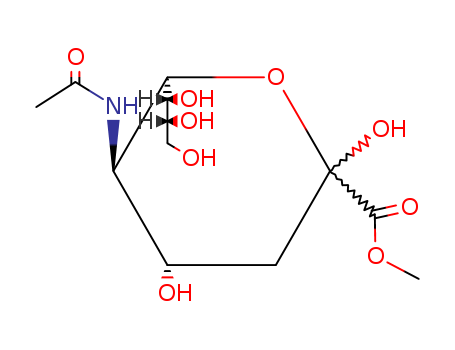
22900-11-4
- Product Name:N-Acetyl-β-neuraminic acid methyl ester
- Molecular Formula:C12H21 N O9
- Purity:99%
- Molecular Weight:323.3
Product Details
pd_meltingpoint:193.5-194.5°C
Purity:99%
Buy Reliable Quality 22900-11-4 Low Price, Best Quality N-Acetyl-β-neuraminic acid methyl ester
- Molecular Formula:C12H21NO9
- Molecular Weight:323.3
- Vapor Pressure:2.86E-22mmHg at 25°C
- Melting Point:193.5-194.5°C
- Refractive Index:-27.5 ° (C=1, H2O)
- Boiling Point:693.8°C at 760 mmHg
- PKA:10.40±0.70(Predicted)
- Flash Point:373.4°C
- PSA:165.78000
- Density:1.51g/cm3
- LogP:-3.39250
N-ACETYLNEURAMINIC ACID METHYL ESTER(Cas 22900-11-4) Usage
|
Chemical Properties |
White to Off-White Solid |
|
Uses |
Derivative of Neuraminic Acid |
InChI:InChI=1/C12H21NO9/c1-5(15)13-8-6(16)3-12(20,11(19)21-2)22-10(8)9(18)7(17)4-14/h6-10,14,16-18,20H,3-4H2,1-2H3,(H,13,15)/t6-,7+,8+,9+,10+,12-/m0/s1
22900-11-4 Relevant articles
Quantitative Standards of 4-O-Acetyl- and 9-O-Acetyl-N-Acetylneuraminic Acid for the Analysis of Plasma and Serum
Cheeseman, Jack,Badia, Concepcion,Thomson, Rebecca I.,Kuhnle, Gunter,Gardner, Richard A.,Spencer, Daniel I. R.,Osborn, Helen M. I.
, (2022/01/20)
N-Acetylneuraminic acid (sialic acid, Ne...
One pot synthesis of thio -glycosides via aziridine opening reactions
Hribernik, Nives,Tamburrini, Alice,Falletta, Ermelinda,Bernardi, Anna
supporting information, p. 233 - 247 (2021/01/14)
A one-pot aziridine opening reaction by ...
Revealing Functional Significance of Interleukin-2 Glycoproteoforms Enabled by Expressed Serine Ligation
Cao, Qi,Li, Bin,Liu, Jiazhi,Liu, Lizhen,Liu, Xinnan,Shao, Hong,Tao, Houchao,Wang, Can,Wang, Ping,Xue, Dongxiang,Ye, Farong,Yu, Biao,Zhao, Hongbo,Zhao, Jie
supporting information, (2022/01/31)
Naturally occurring interleukin-2 (IL-2)...
Addition of Sialic Acid to Insulin Confers Superior Physical Properties and Bioequivalence
Kabotso, Daniel E. K.,Kabotso, Daniel E. K.,Smiley, David,Mayer, John P.,Gelfanov, Vasily M.,Perez-Tilve, Diego,Dimarchi, Richard D.,Pohl, Nicola L. B.,Liu, Fa
, p. 6134 - 6143 (2020/07/10)
Native insulin is susceptible to biophys...
22900-11-4 Process route
-
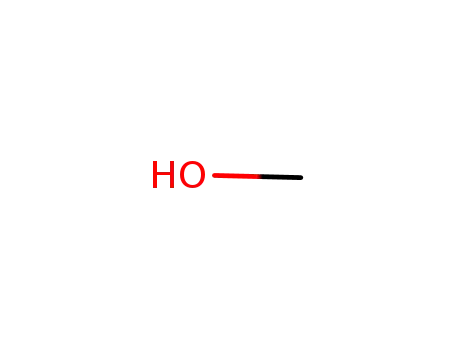
- 67-56-1
methanol

-
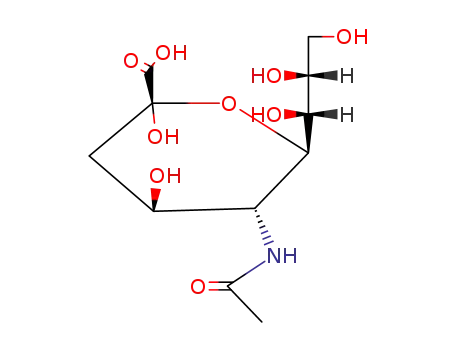
- 489-46-3,6813-81-6,14120-72-0,19342-33-7,21646-00-4,82349-00-6,99395-98-9,99395-99-0,99396-00-6,102734-10-1,140671-47-2,140850-42-6,140850-43-7,140850-44-8,145375-13-9
sialic acid

-
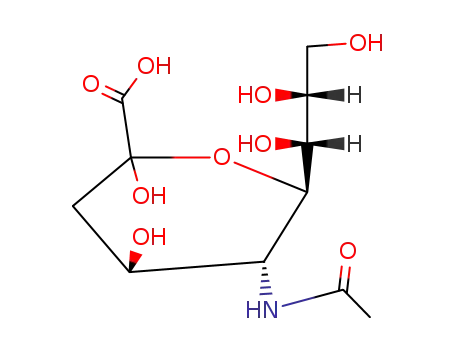
- 22900-11-4,100678-43-1,109121-98-4,109122-00-1,119241-60-0,119241-61-1,119241-62-2,20298-35-5
(2R,3R,4S)-3-acetamido-4-hydroxy-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-dihydro-2H-pyran-6-carboxylic acid
| Conditions | Yield |
|---|---|
|
With IR(H+)resin; at 20 ℃; Inert atmosphere;
|
100% |
|
With trifluoroacetic acid; at 20 ℃;
|
93.8% |
|
With hydrogenchloride; In water; at 50 ℃; for 2.5h;
|
86.5% |
|
With Dowex 50W X-8 (H+) cation-exchange resin; at 20 ℃; for 16h;
|
75% |
|
With acetyl chloride; at 0 - 20 ℃;
|
|
|
With Dowex H+;
|
|
|
With trifluoroacetic acid;
|
|
|
With H+-exchange resin; at 20 ℃; for 40h;
|
|
|
at 20 ℃; for 48h; Inert atmosphere;
|
-

- 67-56-1
methanol

-
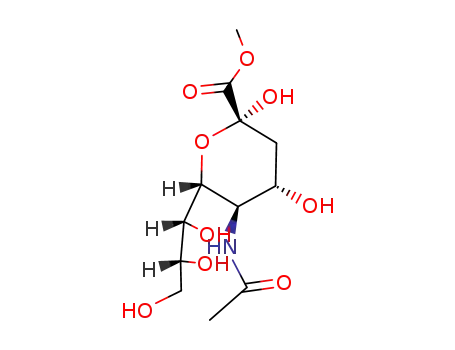
- 6813-81-6,14120-72-0,19342-33-7,21646-00-4,82349-00-6,99395-98-9,99395-99-0,99396-00-6,102734-10-1,489-46-3,140671-47-2,140850-42-6,140850-43-7,140850-44-8,145375-13-9
N-acetyl neuraminic acid

-
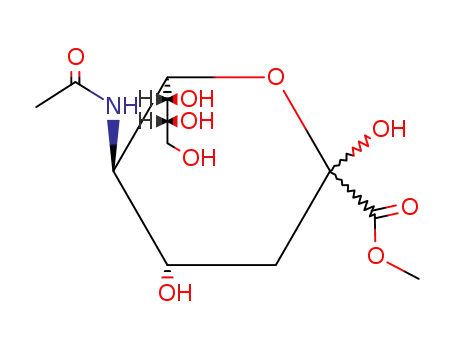
- 20298-35-5,22900-11-4,100678-43-1,109121-98-4,109122-00-1,119241-60-0,119241-61-1,119241-62-2
N-acetylneuraminic acid methyl ester
| Conditions | Yield |
|---|---|
|
With IR120(H+); for 15h;
|
100% |
|
With Amberlite IR120 (H+);
|
100% |
|
With Amberlite IR-120 (H+) resin; at 20 ℃; for 16h;
|
100% |
|
With Dowex H+ resin; at 80 ℃; for 1h; Microwave irradiation;
|
100% |
|
With Amberlite IR120 H+; at 20 ℃;
|
100% |
|
With dowex; at 20 ℃; for 24h;
|
100% |
|
With trifluoroacetic acid;
|
99% |
|
With trifluoroacetic acid; at 20 ℃; for 48h;
|
94% |
|
at 80 ℃; for 0.25h; Microwave irradiation;
|
94% |
|
With DOWEX-50W-X2; at 20 ℃; for 24h;
|
92% |
|
With Dowex-50 W-X2; at 21 ℃; for 24h; Inert atmosphere;
|
92% |
|
With trifluoroacetic acid; at 23 ℃; for 48h;
|
87% |
|
With DOWEX50WX8; for 4h; Inert atmosphere;
|
85% |
|
With Amberlite IR120; at 20 ℃; Inert atmosphere;
|
85% |
|
With trifluoroacetic acid; for 24h; Ambient temperature;
|
59% |
|
Dowex 50W (H+);
|
|
|
With hydrogen cation;
|
|
|
With Dowex H+; at 20 ℃; for 24h;
|
|
|
H+ resin; at 20 ℃;
|
|
|
With hydrogen cation;
|
|
|
With Amberlyst 15; at 20 ℃; for 16h;
|
|
|
Inert atmosphere; Acidic conditions;
|
|
|
With amberlite-H+;
|
|
|
Dowex-50 W-X2; at 21 ℃; for 24h; Inert atmosphere;
|
|
|
With Dowex-50WX2 resin (H+ form); at 20 ℃; for 18h; Inert atmosphere;
|
|
|
With H+ resine;
|
|
|
With Dowex 50WX4 (H+) resin; at 20 ℃; for 20h;
|
22900-11-4 Upstream products
-
186581-53-3

diazomethane
-
489-46-3
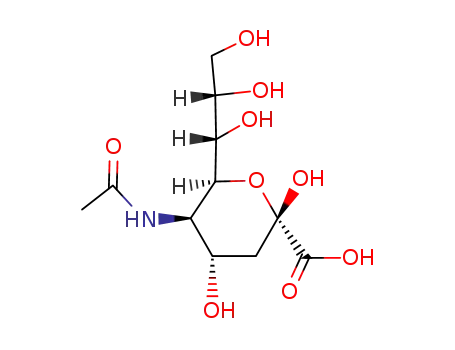
N-acetylneuraminic acid
-
67-56-1

methanol
-
6813-81-6

N-acetyl neuraminic acid
22900-11-4 Downstream products
-
20298-34-4
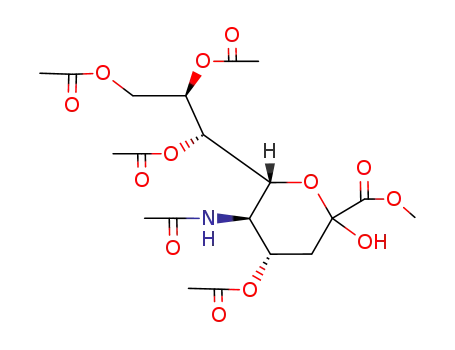
methyl (5-acetamido-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-β-D-glycero-D-galacto-2-nonulopyranosyl)onate
-
67670-69-3
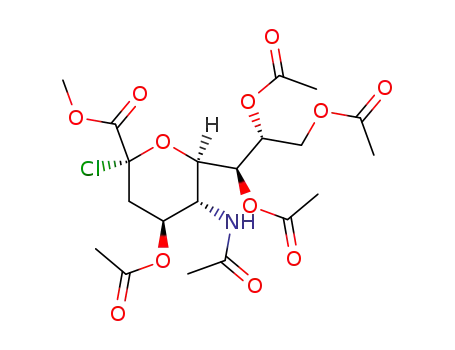
N-acetyl-4,7,8,9-tetra-O-acetyl-2-chloro-2-deoxyneuraminic acid methyl ester
-
132883-18-2
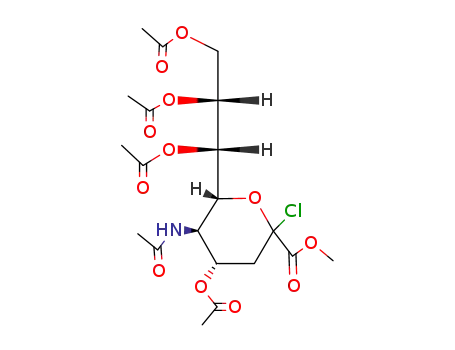
methyl 5-acetamido-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-D-glycero-β-D-galacto-non-2-ulopyranosyl chloride
-
144240-36-8
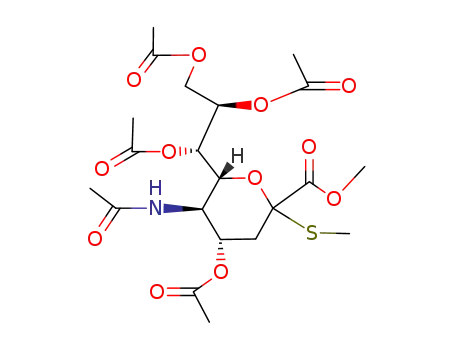
methyl (methyl 5-acetamido-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-2-thio-D-glycero-D-galacto-2-nonulopyranosid)onate
Relevant Products
-
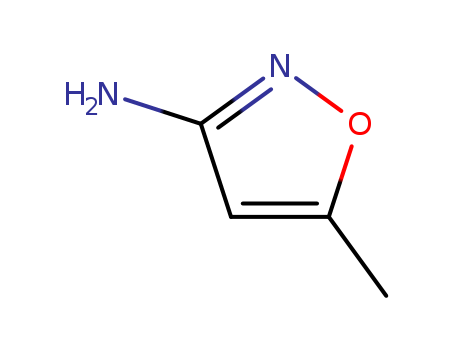
3-Amino-5-methylisoxazole
CAS:1072-67-9
-
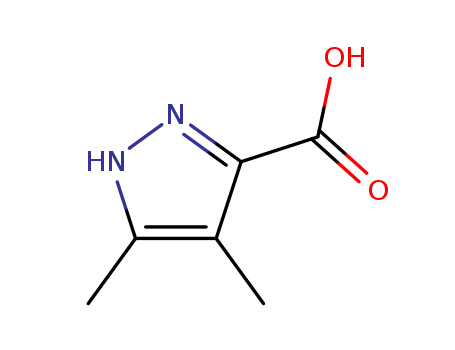
3,4-Dimethylpyrazole-5-carboxylic Acid
CAS:89831-40-3