219725-67-4
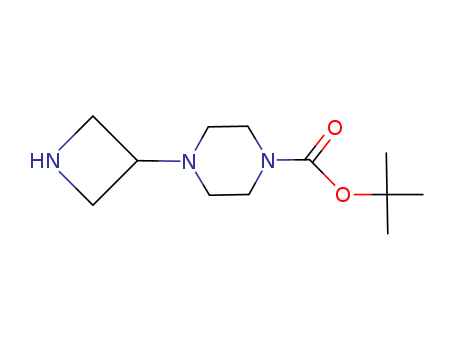
- Product Name:tert-Butyl 4-(azetidin-3-yl)piperazine-1-carboxylate
- Molecular Formula:C12H23N3O2
- Purity:99%
- Molecular Weight:241.334
Product Details
Purity:99%
Quality Factory Supply 99% Pure tert-Butyl 4-(azetidin-3-yl)piperazine-1-carboxylate 219725-67-4 Competitive Price
- Molecular Formula:C12H23N3O2
- Molecular Weight:241.334
- Vapor Pressure:0.000106mmHg at 25°C
- Boiling Point:337.3oC at 760 mmHg
- PKA:10.81±0.40(Predicted)
- Flash Point:157.8oC
- PSA:44.81000
- Density:1.115g/cm3
- LogP:0.71550
TERT-BUTYL 4-(AZETIDIN-3-YL)PIPERAZINE-1-CARBOXYLATE(Cas 219725-67-4) Usage
|
General Description |
TERT-BUTYL 4-(AZETIDIN-3-YL)PIPERAZINE-1-CARBOXYLATE is a chemical compound with the molecular formula C14H24N4O2. It is a tert-butyl ester derivative of piperazine-1-carboxylic acid, with a substituted azetidinyl group at the 4-position of the piperazine ring. TERT-BUTYL 4-(AZETIDIN-3-YL)PIPERAZINE-1-CARBOXYLATE is a potential pharmaceutical intermediate with various potential applications, such as in the synthesis of drug molecules and other organic compounds. It is important to handle and use this chemical with proper care and adherence to safety guidelines due to its potential hazards and reactivity. |
InChI:InChI=1/C12H23N3O2/c1-12(2,3)17-11(16)15-6-4-14(5-7-15)10-8-13-9-10/h10,13H,4-9H2,1-3H3
219725-67-4 Relevant articles
RAPIDLY ACCELERATING FIBROSARCOMA PROTEIN DEGRADING COMPOUNDS AND ASSOCIATED METHODS OF USE
-
Paragraph 00276, (2022/03/22)
Bifunctional compounds, which find utili...
Compound with degrading STAT3 enzyme and preparation method and pharmaceutical application thereof
-
Paragraph 0426; 0429; 0435-0439, (2021/10/27)
The present invention relates to a compo...
POLYCYCLIC COMPOUNDS AND METHODS FOR THE TARGETED DEGRADATION OF RAPIDLY ACCELERATED FIBROSARCOMA POLYPEPTIDES
-
Paragraph 1276; 1277, (2020/03/29)
The present disclosure relates to bifunc...
COMBINATION THERAPIES FOR TREATMENT OF CANCER
-
Paragraph 319; 320, (2016/04/09)
Combination therapies for treatment of c...
219725-67-4 Process route
-
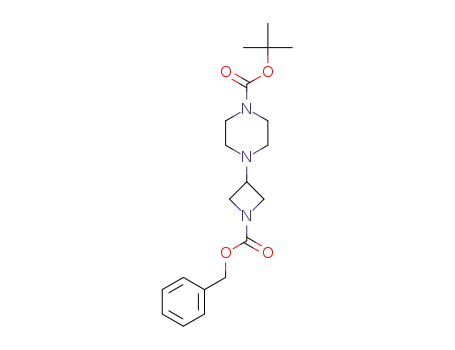
- 1245646-73-4
tert-butyl 4-(1-((benzyloxy)carbonyl)azetidin-3-yl)piperazine-1-carboxylate

-
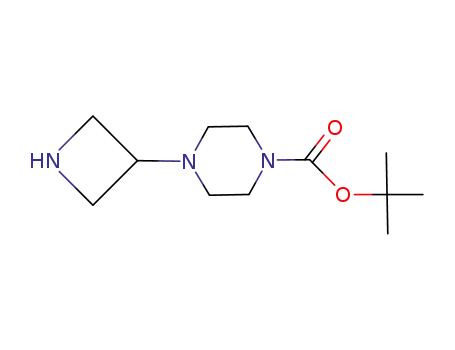
- 219725-67-4
tert-butyl 4-(azetidin-3-yl)piperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; methanol; at 30 ℃; for 12h; under 2585.81 Torr;
|
92% |
|
With palladium 10% on activated carbon; hydrogen; In tetrahydrofuran; methanol; at 30 ℃; for 12h; under 2585.81 Torr;
|
92% |
|
With palladium on activated charcoal; hydrogen; In methanol; at 20 ℃; for 2h;
|
91.79% |
|
With palladium on activated charcoal; hydrogen; In methanol; at 20 ℃; for 5h;
|
90.8% |
-
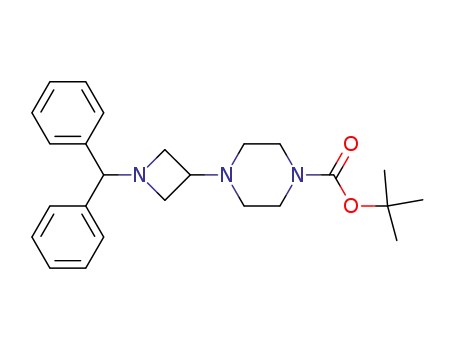
- 178311-82-5
tert-butyl 4-(1-benzhydrylazetidin-3-yl)piperazine-1-carboxylate

-

- 219725-67-4
tert-butyl 4-(azetidin-3-yl)piperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 50 ℃; for 48h;
|
|
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 50 ℃; for 48h;
|
219725-67-4 Upstream products
-
178311-82-5

tert-butyl 4-(1-benzhydrylazetidin-3-yl)piperazine-1-carboxylate
-
57260-71-6
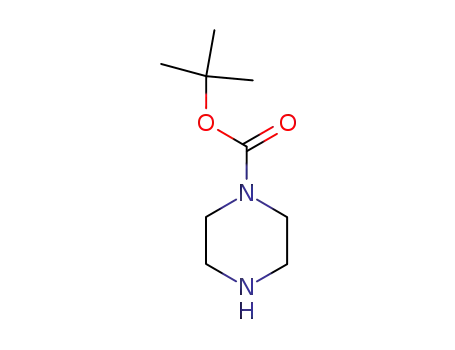
1-t-Butoxycarbonylpiperazine
-
1245646-73-4

tert-butyl 4-(1-((benzyloxy)carbonyl)azetidin-3-yl)piperazine-1-carboxylate
Relevant Products
-
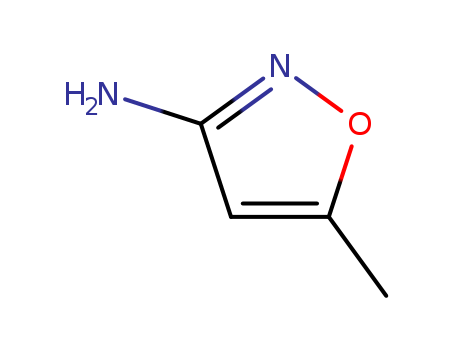
3-Amino-5-methylisoxazole
CAS:1072-67-9
-
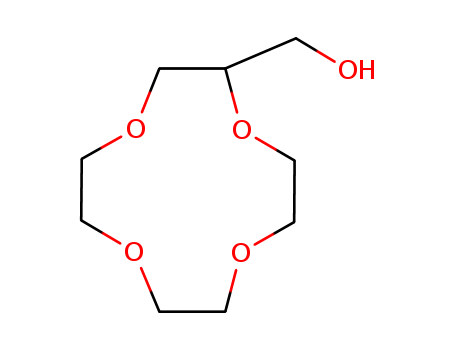
2-(Hydroxymethyl)-12-crown 4-Ether
CAS:75507-26-5