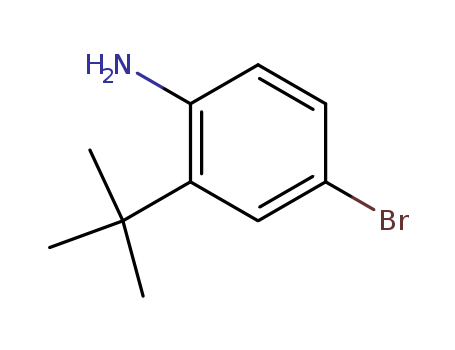
850012-44-1
- Product Name: 4-bromo-2-tert-butylphenylamine
- Molecular Formula:C10H14BrN
- Purity:99%
- Molecular Weight:228.132
Product Details
Purity:99%
Chinese Manufacturer Supply Top Purity 4-bromo-2-tert-butylphenylamine 850012-44-1 Competitive Price
- Molecular Formula:C10H14BrN
- Molecular Weight:228.132
- Boiling Point:146-147 °C(Press: 9 Torr)
- PKA:3.08±0.10(Predicted)
- PSA:26.02000
- Density:1.306±0.06 g/cm3(Predicted)
- LogP:3.91000
850012-44-1 Relevant articles
New method for the synthesis of N-tert-alkoxyarylaminyl radicals
Miura, Yozo,Muranaka, Yoshikazu,Teki, Yoshio
, p. 4786 - 4794 (2006)
The reactions of 2,4-diaryl-6-tert-butyl...
A Photorobust Mo(0) Complex Mimicking [Os(2,2′-bipyridine)3]2+and Its Application in Red-to-Blue Upconversion
Bilger, Jakob B.,Kerzig, Christoph,Larsen, Christopher B.,Wenger, Oliver S.
supporting information, p. 1651 - 1663 (2021/02/01)
Osmium(II) polypyridines are a well-know...
KRAS G12C INHIBITORS AND METHODS OF USING THE SAME
-
Paragraph 0255; 0256, (2019/11/22)
Provided herein are KRAS G12C inhibitors...
HORMONE RECEPTOR MODULATORS FOR TREATING METABOLIC CONDITIONS AND DISORDERS
-
Page/Page column 392; 393, (2018/03/25)
The invention relates to activators of F...
Iron-Catalyzed Intramolecular Aminations of C(sp3)?H Bonds in Alkylaryl Azides
Alt, Isabel T.,Guttroff, Claudia,Plietker, Bernd
supporting information, p. 10582 - 10586 (2017/08/22)
The nucleophilic iron complex Bu4N[Fe(CO...
850012-44-1 Process route
-
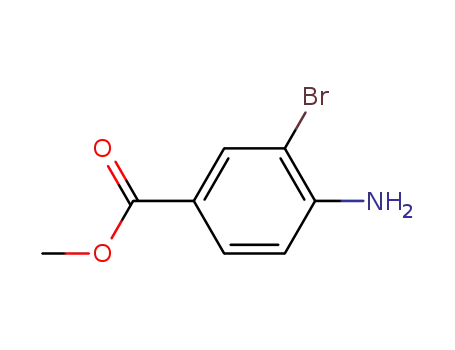
- 106896-49-5
methyl 4-amino-3-bromobenzoate

-
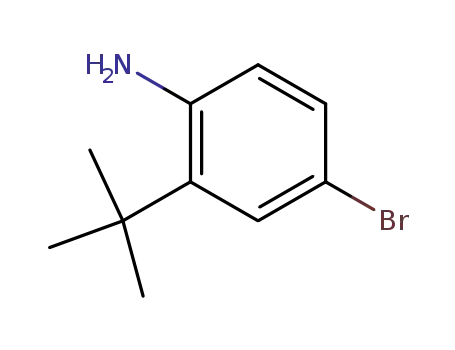
- 850012-44-1
4-bromo-(2-tert-butyl)aniline
| Conditions | Yield |
|---|---|
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 0 - 20 ℃;
|
84% |
-
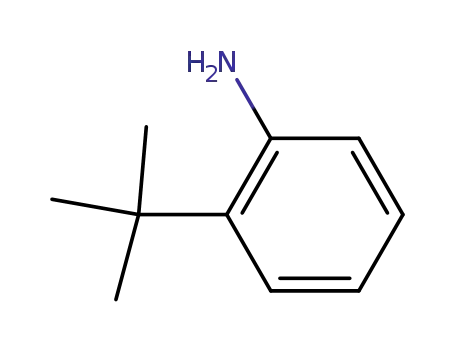
- 6310-21-0
2-(1,1-dimethylethyl)-benzenamine

-

- 850012-44-1
4-bromo-(2-tert-butyl)aniline
| Conditions | Yield |
|---|---|
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 0 - 20 ℃; for 0.166667h;
|
100% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 5 ℃; for 1.25h; Product distribution / selectivity;
|
100% |
|
With tetrabuthylammonium tribromide; In tetrahydrofuran; at 0 ℃; for 0.5h;
|
98% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 0 ℃; for 0.666667h; Inert atmosphere;
|
93% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 1h; Product distribution / selectivity;
|
83% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃;
|
83% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 4h;
|
79% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 4h;
|
79% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 4h;
|
79% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
79% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
78% |
|
2-(1,1-dimethylethyl)-benzenamine; With hydrogen bromide; In water; acetic acid; dimethyl sulfoxide; at 0 - 20 ℃; for 4h;
With sodium hydroxide; In water; acetic acid; dimethyl sulfoxide; ethyl acetate; pH=8;
|
71% |
|
2-(1,1-dimethylethyl)-benzenamine; With hydrogen bromide; In water; acetic acid; dimethyl sulfoxide; at 0 - 20 ℃;
With sodium hydroxide; In water; acetic acid; dimethyl sulfoxide; pH=10; Cooling with ice;
|
65% |
|
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 0 - 20 ℃; for 0.166667h;
|
61% |
|
With dihydrogen peroxide; ammonium bromide; acetic acid; for 48h; Inert atmosphere;
|
60% |
|
With dihydrogen peroxide; ammonium bromide; In acetic acid; for 48h;
|
59% |
|
|
|
|
With benzyltrimethylammonium tribromide; In methanol; dichloromethane; at 20 ℃;
|
|
|
Multi-step reaction with 3 steps
1: ethyl acetate / 16 h / 77 °C
2: bromine; acetic acid / 48 h / 20 °C
3: hydrogenchloride / water; isopropyl alcohol / 18 h / Reflux
With hydrogenchloride; bromine; acetic acid; In water; ethyl acetate; isopropyl alcohol;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃;
|
850012-44-1 Upstream products
-
7402-70-2
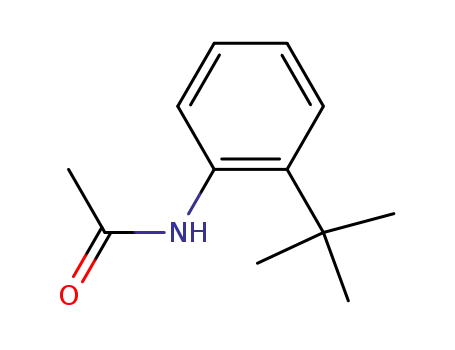
N-(2-tert-butylphenyl)acetamide
-
6310-21-0

2-(1,1-dimethylethyl)-benzenamine
-
20442-98-2

1-azido-2-tert-butylbenzene
-
73621-42-8
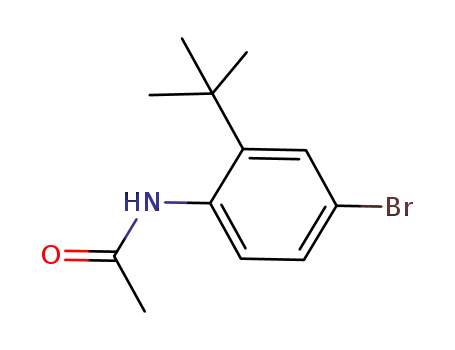
N-(4-bromo-2-tert-butyl-phenyl)-acetamide
850012-44-1 Downstream products
-
923547-56-2
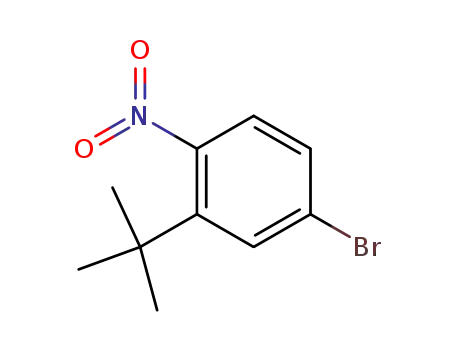
4-bromo-2-(tert-butyl)nitrobenzene
-
70634-28-5
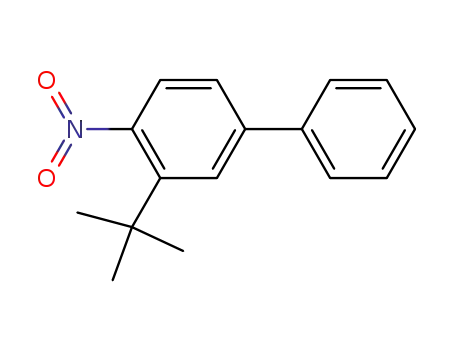
3-(tert-butyl)-4-nitrobiphenyl
-
895543-24-5
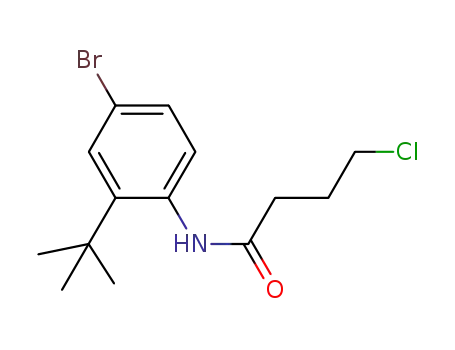
N-(2-tert-butyl-4-bromophenyl)-4-chlorobutanamide
-
1219832-04-8
4-bromo-2-t-butyl-6-iodoaniline
Relevant Products
-
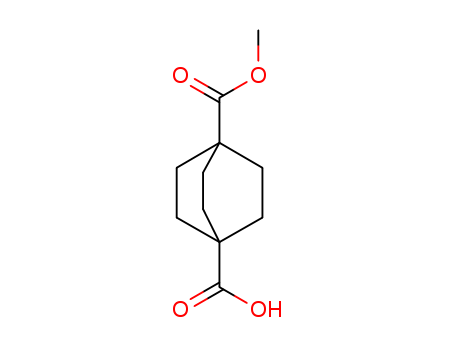
4-(Methoxycarbonyl)bicyclo[2.2.2]octane-1-carboxylic acid
CAS:18720-35-9